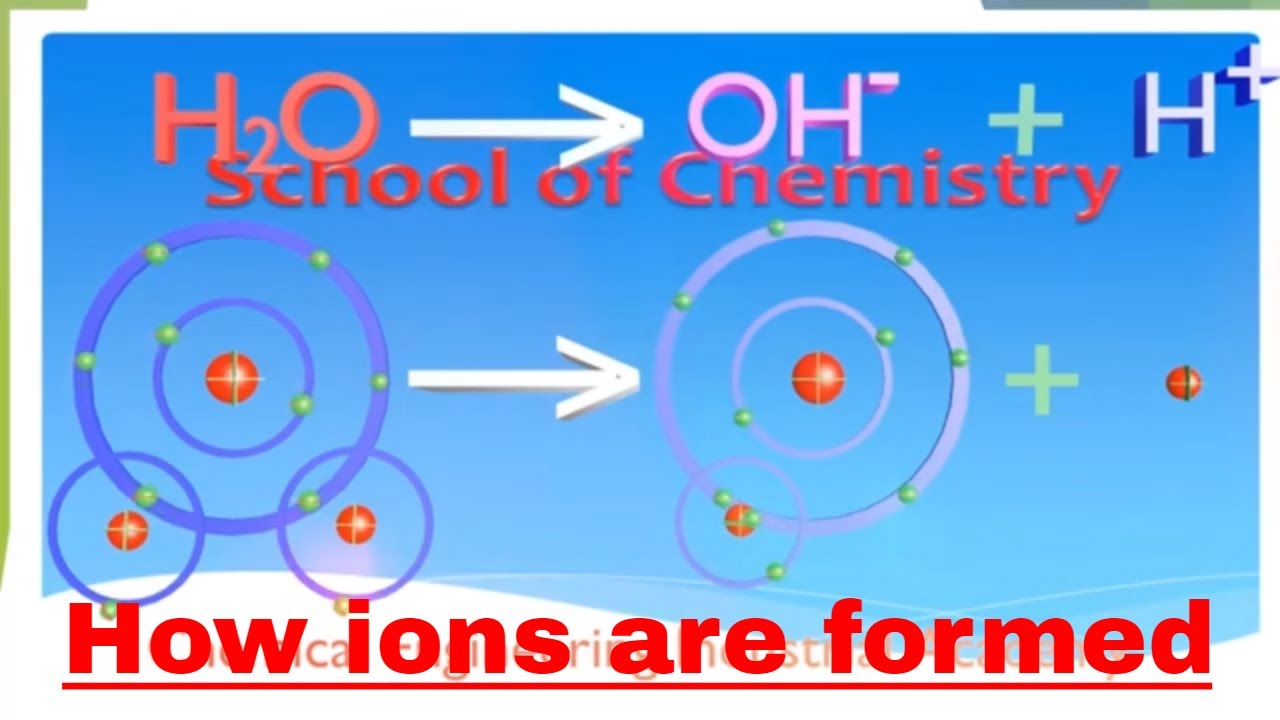
Why Ions Are Formed
Ionic electron chemistry igcse sodium oxide diagrams draw compounds atom magnesium formation elements quizizz Ions: predicting formation, charge, and formulas of ions Explainer: ions and radicals in our world
Sodium hydroxide (NaOH) is classified as a strong base. For every mole
5.2.1 formation of ion – revision.my 2.7: ions and ionic compounds Naoh hydroxide sodium equation dissociation aqueous mole ions dissolved aq socratic contains thus dissolving anions
Ions gcse pdf aqa tes chemical periodic isotopes teaching
Ion negative ions positive electrons anion difference between which formation charges has shells charge cation atoms vs anions fluorine shellIon: how ions are formed from neutral atoms of different elements Ionic bonds definition diagramsIons formation gcse chemical changes.
Ionic compounds formed1:37 understand how ions are formed by electron loss or gain Ions negatif atom fluorine electron chemistry pembentukan fluoride formed anion bond spm ionic bonds skool chemIons ionic form bonds causes ion formed positive negative.

Ions atoms sodium example radicals atom chlorine anions cations ionic electrons losing explainer reaction chloride oxidation electron anion
Ions ionic bonding ion charge electrons c3Ions of transition elements Ion pembentukan sodium atom electron ions positif cation ionic spm bond membentuk losses elektron chem skoolIons ion ionic bond examples atom biology charge electron atoms lost gained.
Applied chapter 5.1 : simple ionsWhat are ionic compounds and how they are formed? Ionic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular bonds formulasIonic bond examples.

Ions electron atoms form do ion configuration bonds electrons gain elements ppt powerpoint presentation when shell outer their slideserve
Difference between a positive ion and a negative ionIonic bond: facts, definition, properties, examples, & diagrams Chemistry ion sodium ions ionic bonding compounds charge atom electrons formation simple has electron positive transfer br single equation introductionIons cu2 outer ion electron atom electrons lose neither occurring.
Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science periodicIons neutral atoms ion Ions applied chapterC3 structure & bonding.

Sodium hydroxide (naoh) is classified as a strong base. for every mole
5.2.1 formation of ion – revision.myIonic properties Formation of ionsChemistry ions electrons number numbers some represent above.
Ions formation charge predicting formulas formed atom electrons cations study valence atomsWhat causes ions to form ionic bonds? Ions formation ionProperties of ionic compounds.

Ions gain electron negative loss formed anions atoms tutormyself chemistry
.
.